What is exactly the maximum medical therapy tolerated by patients?

João Filipe, MD
Introdução
Introduction
Glaucoma is a progressive multifactorial optic neuropathy, related to the intraocular pressure (IOP).1 To date, IOP is precisely the only risk factor whose modification is widely accepted to be effective in reducing the progression of glaucoma.1
Thus, if on the one hand we have several drugs that help us reduce IOP, on the other hand, we need to have the good sense to consider the cost, side effects and the compliance of the patient to the chronic medication. In other words, what is the maximum medical therapy tolerated by the patient?
The concept of maximum medical therapy can be defined as the reduction of IOP with the use of the largest number of drugs available and tolerated by the patient, the patient’s compliance being relevant here when the medication is increased.2
Scientific Evidence
Briefly, the goal of the glaucoma treatment (whether medical or surgical) is to halt the progression of glaucoma, maintain visual function and the quality of life at an acceptable cost.3 For this purpose, we should reduce IOP to a given target IOP.2 However, there is no universal target IOP.2,3,4
Thus, there are some factors suggested by the European Society of Glaucoma (EGS)3 that should determine lower target IOP (and, consequently, greater aggressiveness of the treatment), such as: greater severity of the glaucoma, higher rate of progression (determined during ophthalmic observations), lower IOP value without treatment, lower age, higher number of other risk factors for glaucoma. Medical therapy remains the most common initial method for reducing IOP3,5. Several drugs have emerged over the years to enrich the available medical therapy - scheme 1.
To achieve the target IOP, the type and number of ocular hypotensive drugs we should use is also specified in the EGS Guidelines.3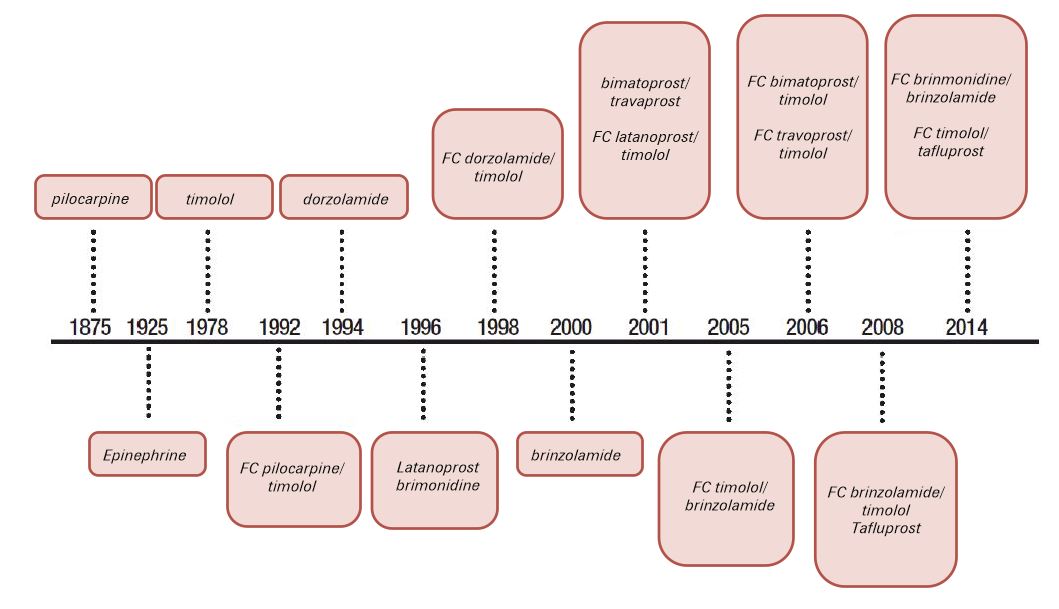
Systemic carbonic anhydrase inhibitors are available since 1995
Therefore, the following prescription procedures are recommended:
1. Use in monotherapy a 1st line drug (1 of 4 therapeutic classes: beta-blocker, prostaglandin or prostamide analogue, topical carbonic anhydrase inhibitor or selective alpha-2 agonist)
2. Identify whether monotherapy was effective (total or partial achievement of target IOP) and whether it was tolerated:
a. Fully effective (reduced IOP to target IOP): maintain and monitor with complementary exams
b. Totally ineffective (did not reduce IOP): replace the previous drug with other 1st line drug from another therapeutic class
i. Check again for IOP reduction:
1. Reached target IOP: maintain and monitor
2. Did not reach target IOP: change monotherapy again or consider other options (namely combination therapy, laser, surgery)
c. Partially effective (reduced IOP but did not reach target IOP): associate a 1st line drug from another therapeutic class in a fixed combination (a single bottle in total)
i. Check again for IOP reduction:
1. Reached target IOP: maintain and monitor
2. Reduced IOP but did not reach target IOP: add third first line drug
a. Reassess: if target IOP was not reached: consider other therapeutic options
3. Did not reduce IOP: replace 2nd drug or consider other therapeutic options
It should be noted that if progression of glaucomatous damage is detected despite target IOP being achieved, we will have to lower the target IOP or consider other forms of treatment.
Some examples with drugs commercially available in Portugal (that should be used, according to the ESG Guidelines3, not as 1st or 2nd line):
· Fixed combination of prostaglandin (PG) with beta-blocker (BB)
o Associated with brimonidine
or
· Fixed combination of PG with BB
o Associated with dorzolamide or brinzolamide
or
· Fixed combination of brimonidine with BB
o Associated with PG
or
· Fixed combination of brimonidine with BB
o Associated with dorzolamide or brinzolamide
or
· Fixed combination of dorzolamide with BB
o Associated with PG
or
· Fixed combination of dorzolamide with BB
o Associated with brimonidine
or
· Fixed combination of brinzolamide with BB
o Associated with PG
or
· Fixed combination of brinzolamide with BB
o Associated with brimonidine
or
· Fixed combination of brinzolamide with brimonidine (latest market fixed combination)
o Associated with BB
or
· Fixed combination of brinzolamide with brimonidine
o Associated with prostaglandin
Another remark relates to polymedication2: the more drugs are used, the greater the risk of intolerance and poor compliance1 on the part of the patient, which reduces the therapeutic efficacy.
In particular cases (such as extremely elderly patients, patients whose ocular hypertension is associated with an ophthalmologic temporary pathological condition, or prior to surgery), we may also resort to a “4th therapeutic line”:
· Associating the maximum medical therapy with an ocular hypotensive from another pharmacological group
o For example, if the patient is taking a fixed combination of prostaglandin with timolol associated with brimonidine, we can also add a carbonic anhydrase inhibitor (brinzolamide or dorzolamide). The fixed combination of brinzolamide with brimonidine associated with a fixed combination of prostaglandin with BB is currently the form of maximum therapy available to achieve the target IOP in each patient. We often use the mnemonic of "two vials, four drugs", allowing greater comfort in the number of applications of eye drops.
o Associating oral medication (acetazolamide, from 250 to 1000mg per day, according to several factors).
o Note that that acetazolamide is responsible for several side effects; its chronic use should be avoided except in highly selected cases and with regular monitoring of laboratory parameters, such as in the pre-surgical period.
· In hospitalized patients and for short intervals (days), we also have the “5th line”: mannitol 20% intravenous. Mannitol is a hyperosmotic agent, useful when we need to reduce considerably and quickly the IOP.
The use of fixed combinations allows:6
· reduction of the washout effect
· a lower number of vials and applications, leading to greater compliance
· a lower exposure to ocular preservatives
· minimization of costs.
Approaches with neuroprotective agents have been explored. Although preclinical findings suggest a potential neuroprotective role, clinical studies with these oral or topical agents were inconclusive.7 Despite the improvement in the formulations and the emergence of new fixed combinations, no new pharmacological class was recently presented for the medical treatment of glaucoma.7 Several molecules are being studied in clinical trials that may become promising classes in the glaucoma therapy: ρ-kinase inhibitor8, adenosine receptor agonists9, latrunculin B10, modified prostaglandin analogs .11
Finally, we would like to highlight that medicine is an art that, despite having several therapeutic strategies, has to identify for each patient which treatment is the most appropriate. For example, in a patient with a progressive glaucoma due to poor compliance, increasing the number of vials will only contribute to further reducing compliance, with subsequent progression worsening.
Likewise, we should be very cautious with patients who tell us they do not have time to put the drops, or that all drops sting, making an effort to prescribe few drugs and adapting the concept of maximum medical therapy to each particular patient...
We must adjust the therapeutic weapons available for each clinical case, and this concept is encompassed in the maximum medical therapy. Some patients have a compliance that is very dependent on the number of drugs we use, abandoning the eye drops when they are numerous. We must be attentive to these cases and prescribe (as far as possible) specifically the number of eye drops that each patient will tolerate.
There are patients whose maximum medical therapy is only one drug because they do not tolerate more. There are others who have no tolerance to any drug, and we have the role of exploring other therapeutic weapons.
Consequently, maximum medical therapy, despite being governed by specific and rigorous guidelines, is a dynamic and flexible concept, adaptable to each patient. It is up to us, as ophthalmologists, to find out what is the maximum medical therapy for the patient facing us, and use it to maximize compliance and prevent the progression of glaucoma in each and every one of our patients.
