Should ocular hypertension be monitored with OCT without perimetry?*

Maria da Luz Freitas, MD, FEBOS Glaucoma
Glaucoma is a chronic, degenerative disease characterized by the loss of retinal ganglion cells leading to characteristic changes in the optic nerve head and the optic fiber layer. Early diagnosis and treatment are critical to prevent permanent structural injury and irreversible visual function loss.
This has been supported for a long time by studies, papers and recommendations, some of which are presented below. The paper published in 2002 in the Archives of Ophthalmology on the Ocular Hypertension Treatment Study (OHTS)1 states that changes in the optic disc are detected earlier than changes in the visual field in approximately half of the patients with ocular hypertension progressing to glaucoma. The ESAFAT2,3 (European Structure and Function Assessment Trial) concluded that when the assessment is conducted by glaucoma specialists, stereoscopic optic disc photograph accuracy is 80.5%, decreasing to 59% when matched with visual field analysis. The September 2013 monthly issue of the European Glaucoma Society was dedicated to the usefulness of measuring nerve fiber layer thickness in determining which patients with ocular hypertension will develop glaucoma: patients with ocular hypertension and moderate to severe loss of nerve fiber layer have a 7- to 8-fold higher associated risk of subsequent visual field loss. Furthermore, nerve fiber layer injuries occur in 60% of eyes with elevated eye pressure 6 years before observable changes in visual field4,5,6,7.
There is a broad consensus up to this point. However, the question to be answered is whether OCT dispenses perimetry in studying and monitoring patients with ocular hypertension. In order to respond to this question, we must know the sensitivity, the specificity and the accuracy of OCT alone in assessing the optic disc, nerve fiber layers and in measuring the ganglion cell layer-inner plexiform layer.
Mwanza et al8,9,10 demonstrated the ability of Cirrus HD OCT to discriminate normal from glaucomatous eyes. Different optic nerve head and nerve fiber layer thickness assessment parameters can differentiate between normal and glaucomatous eyes. This ability is maintained in distinguishing normal from mild glaucomatous eyes, but it is lost in differentiating moderate from severe glaucoma (graphs 1 and 2).
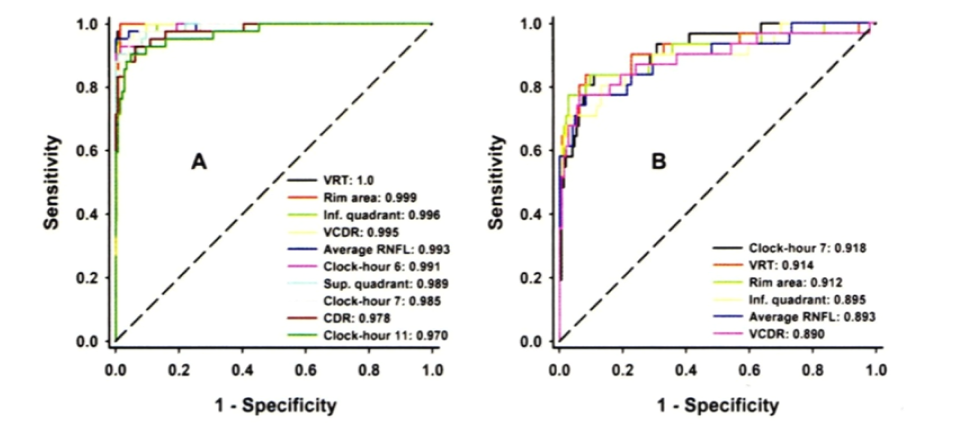
Graph 18 – The ROCs of the best parameters for discriminating between normal and eyes with moderate to severe glaucoma (A) and of the overall best six parameters for discriminating between normal and eyes with mild glaucoma (B). CDR = cup-to-disk ratio; Inf. = inferior; RNFL= retinal nerve fiber layer; ROC= receiver operator characteristic; Sup. = superior; VCDR= vertical cup-to-disk ratio; VRT= vertical rim thickness
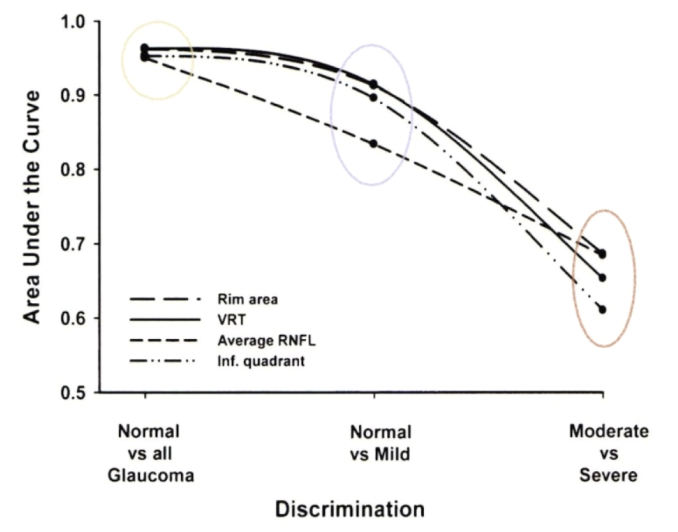
Graph 28 – Trend of the ability of vertical rim thickness (VRT), rim area, average retinal nerve fiber layer (RNFL), and RNFL thickness of the inferior (inf) quadrant to discriminate between normal and glaucomatous eyes, normal and mild glaucoma, and between moderate and severe glaucoma. Inf. = inferior; RNFL= retinal nerve fiber layer; VRT= vertical rim thickness
Mwanza also concluded that there is no difference between optic nerve head and nerve fiber layer thickness assessment parameters in distinguishing normal from glaucomatous eyes. When this working group studied the diagnostic ability of ganglion cell layer-inner plexiform layer thickness, they concluded that it is as good as the optic nerve head and peripapillary nerve fiber layer thickness parameters. However, the sensitivity and positive predictive value of nerve fiber layer in the inferior sector is higher than any other parameter (Table 1).
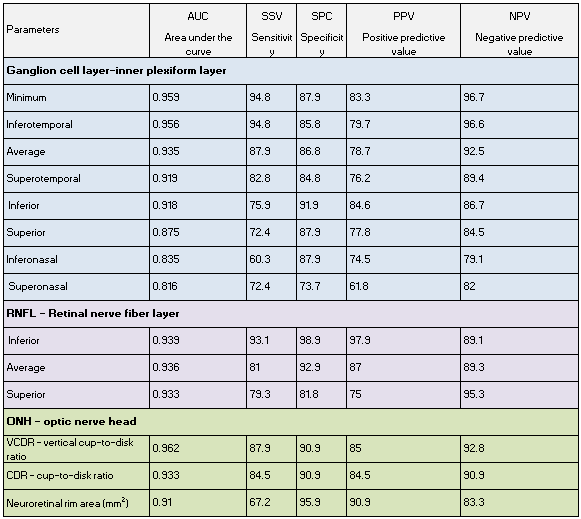
Table 110 – Discriminating ability of macular ganglion cell-inner plexiform layer, peripapillary retinal nerve fiber layer, and optic nerve parameters for early glaucoma
In 2011, Medeiros and co-workers11 published their work, which intended to estimate the ability to predict early development of perimetric changes by counting peripapillary ganglion cells in eyes with suspected glaucoma which showed conversion, when compared to a normal population. They have used several empirical formulas and concluded that there is an average ganglion cell loss of 28.4% before the onset of the first perimetric signs. However, they noted a wide variability among studied eyes.
Shin et al12 assessed the diagnostic ability of ganglion cell-inner plexiform layer thickness according to the location of visual fields loss. They studied eighty-four patients with early glaucoma and 43 normal subjects, which were divided into three different groups: group 1, no perimetric changes; group 2 isolated parafoveal scotoma; and group 3, isolated peripheral nasal step. The average and minimum ganglion cell-inner plexiform layer thickness is significantly lower in group 2 than in group 3. However, there was no significant difference in peripapillary retinal nerve fiber layer thickness between Groups 2 and 3. They have concluded that the diagnostic ability of ganglion cell-inner plexiform layer thickness largely depends on the location of visual fields loss (Figure 1).
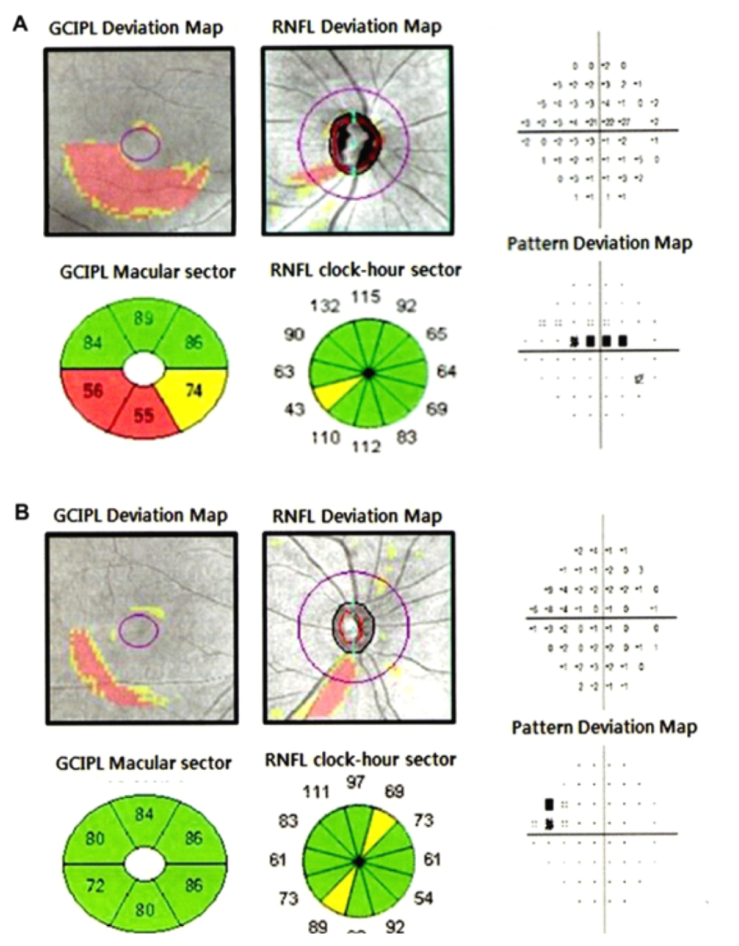
Figure 112 - (A) Case of superior parafoveal scotoma. (B) Case of superior peripheral nasal step.
The January 2014 monthly issue of the European Glaucoma Society suggests that optic nerve head measurement accuracy is still not ideal and that such measurements require further development and optimization 13,14,15,16.
According to the studies presented, sensitivity, specificity and positive predictive value of some parameters (which are not similar for all eyes or types of initial injury) are high. However, the accuracy of assessment methods should be considered together with the issue of the different prospective study designs and population types. Regarding the varying study designs, I would like to mention the criteria used to define initial injury: sometimes, they are subjective and structural, such as photographs; others, objective and structural, but of varying types and parameters; others still, use functional criteria; and at last some studies use mixed criteria. The choice of criteria to define initial injury necessarily conditions results and conclusions. Concerning the study population, the criteria are generally so elective that they include too ideal eyes, leaving behind the wide diversity of optic disc sizes and shapes and other diseases such as common maculopathies (for instance, degenerative and diabetic) and other neuropathies. In our clinical practice, this results in lower specificity and positive predictive value, and thus in lower diagnostic performance.
The other issue when following patients with ocular hypertension without perimetry is the ability that OCT has to assess progression and, in this case, conversion of hypertensive into glaucomatous patients. Leung et al17, 18 showed the impact of age on the assessment of peripapillary nerve fiber layer thickness, as well as the impact of age-related changes on the assessment of progression in eyes with glaucoma. With age and per year, the change in average nerve fiber layer thickness is -0.52 mm, in the superior quadrant -1.35 mm and in the inferior quadrant -1.25 mm. As for progression assessment, they concluded that none of the parameters studied (ganglion cell and inner plexiform layer, inner retina, outer retina, total macular thicknesses, and peripapillary nerve fiber layer) had more than a 50% ability to assess progression, which decreased to 26.7% after adjusting for age-related changed. They have also observed that the parameter showing less age-related changes is the peripapillary nerve fiber layer thickness. On the other hand, the concordance between the progression of nerve fiber layer thickness and macular measures was poor, regardless of adjustment for age-related changes.
Na et al19 studied the trend analysis sensitivity of the nerve fiber layer thickness, ganglion cell-inner plexiform layer thickness, and total macular thickness. They saw that the sensitivity of each parameter was 8%, 14% and 5%, respectively (quite a lot lower than progression references when obtained with optic nerve photographs or perimetry).
In conclusion, despite the technological advances and due to the diversity of optic discs, it is hard to establish which structural parameter(s) per se are most recommended in assessing and following eyes with suspected glaucoma and glaucomatous eyes, alone and exclusively. Therefore, I believe that the approach for hypertensive, glaucomatous eyes should be based on a combination of structural and functional investigations, keeping in mind clinical history, risk factor assessment and other differential diagnoses.
* presented at the 5th World Congress COPHy, 20–23 March 2014
