Are generics and brand-name medicines the same?*

Pedro Corsino Fernández Vila, MD
Generic medicines
A generic medicine is defined as a product having the same qualitative and quantitative composition in active ingredients, as well as the same pharmaceutical form, as the reference medicine and whose bioequivalence has been shown by adequate bioavailability studies.
Bioavailability is defined as the amount and speed at which the active ingredient is absorbed from a pharmaceutical form and reaches the site of action (biophase). Bearing in mind that an active ingredient is in balance between systemic circulation and the site of action, it is assumed that drug values in blood represent drug bioavailability. Bioavailability is assessed using pharmacokinetic parameters, including: the area under the curve (AUC), maximum concentration (Cmax) and time to maximum concentration (Tmax).
Bioequivalence is the comparison between the bioavailability of a medicine under study and the bioavailability of a reference drug. It is accepted that a study product is bioequivalent to the reference product when its values (especially the AUC) are within the 90% confidence interval (80–125%).
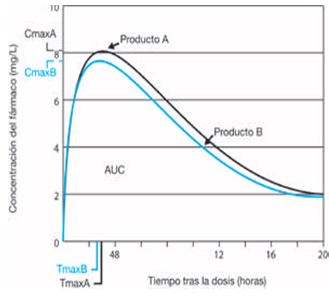
To conduct bioequivalence studies, blood is collected at several time points after administering the product, according to the drug's half-life. The concentration of the active ingredient in blood is then measured to estimate bioavailability kinetic values.
Local or topical action medicines
A local or topical action medicine is a product which is applied locally and assumed to act on the site where it is administered. Systemic effects, if any, should be considered as side effects. Local action medicines include dermatological products (such as creams and ointments), inhaled products, eye and ear drops, nasal products and products administered to the oral, vaginal or rectal cavities with a local effect.
The main challenge in the development of generics of local or topical action medicines is that no bioavailability studies can be performed to compare plasma concentration profiles between reference and generic formulation drugs. Since the effect is produced in mucous membranes or the skin, plasma concentrations are either not high enough to allow drug levels to be determined or, if high enough, they are too small and irregular.
For eye drops, nasal sprays and cutaneous solutions, bioequivalence is accepted if the drug is in the same solution (aqueous or oily) and contains the same active ingredient concentration as the reference product. Minor differences in excipient composition are accepted whenever pharmaceutical properties of the study medicine and the reference medicine are identical or essentially similar. Any qualitative or quantitative difference in excipients must be satisfactorily explained in terms of its influence in therapeutic equivalence. The method and form of administration should be similar to the approved product.
The US Food and Drugs Administration (FDA)1 requires that study medicines contain the same active and inactive ingredients at the same concentration as the reference product. No differences in excipient composition are accepted. However, a laboratory may apply for approval of a medicine that differs from the original, identifying and characterizing the differences and providing information to show that differences do not affect drug safety2. Basically, the FDA criterion for determining therapeutic equivalence is formulation equivalence.
Why do generics manufacturers not copy the original medicine exactly? In one word: patents. On average, each medicine comprises five or six patents. To avoid violating patents, generics manufacturers intentionally change preservatives or the pH, although they never really know for sure how the original product is manufactured. Some laboratories manufacture both the original medicine and the generic. Merck&Co., Inc. manufacture the original Cosopt and the corresponding generic, so it can be assumed that at least in such cases the medicines are exactly alike.
Bearing all the above in mind, a key question arises: In face of the European Medicines Agency, why does the FDA require all active and inactive components of a generic ophthalmic medicine to be exactly the same as in the reference product?
The FDA1 considers that: 1) Changing the excipients or inactive components of a medicine can have a significant effect both on drug efficacy and safety. 2) Any changes in preservatives or preservative concentrations may influence corneal penetration of the active ingredient and 3) Any changes in demulcent or tonicity agents can modify a solution's contact time with the corneal surface, and therefore change the absorption of the active ingredient in solution.
Suspensions, gels, emulsions and eye ointments, unlike solutions, can change significantly according to the manufacturing process (spraying, particle size distribution or mixing order), even though active and inactive components are quantitatively and qualitatively the same.
FDA criteria for determining clinical efficacy in these medicines vary according to the pharmacological group and are based on parameters or measures used to establish the efficacy of the innovator drug. For instance, in ocular hypotensive medicines, equivalence is determined if the difference measured between both products is lower than 1.5 mm Hg (95% confidence interval) at each time point. In this case, the clinical trial must include basal intraocular pressure measurements as well as at week 1, 6 and 12, at least at peak and trough times for the active ingredient.
Currently, there are generic ophthalmic medicines for virtually all therapeutic groups. Therefore, there are: anti-infectives, corticosteroids, non-steroidal anti-inflammatory agents (NSAIDs), antiglaucoma drugs, fixed combinations, antiallergic agents, mydriatics and cycloplegics and anaesthetics.
The importance of excipients
Tabla 1. Porcentaje de principio activo y excipientes en diferentes colirios antiglaucomatosos
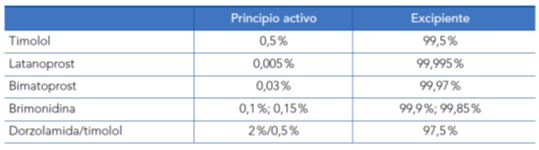
Considering that more than 95% of eye drops are composed of excipients, their importance is obvious. Eye drops contain multiple excipients: 1) a liquid, aqueous (more rarely, oily) vehicle; 2) preservatives; 3) pH-adjusting agents; 4) antioxidants; 5) viscosity agents; 6) buffers. 7) tonicity-adjusting agents4.
The main purpose of preservatives is to inhibit bacterial contamination. Some surfactant preservatives also contribute to keeping lipophilic drugs in solution, such as prostaglandin analogues.
The importance of preservatives is illustrated by replacing one single preservative, BAK, with another, Purite®. This causes the drug to achieve higher levels in the aqueous humour with the same concentration (brimonidine 0.15%), comparable to those of the same drug at a higher concentration (brimonidine-BAK 0.20%)5. The reason is that preservatives such as BAK prevent the pH from increasing beyond 6.7/6.8, whereas the pH can increase up to 7.8 with Purite®. Thus, changing the preservative and increasing the pH improved the corneal penetration of brimonidine and increased the drug's concentration in the aqueous humour.
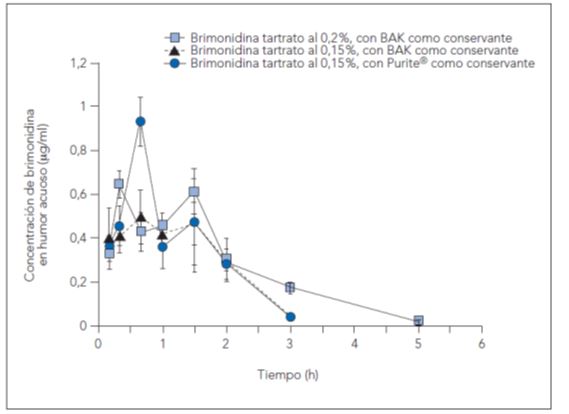
Figura 2. Concentraciones de brimonidina en humor acuoso en el tiempo.
Fuente: Dong JQ, Babusis DM, Welty DF, Acheampong AA, Tang-Liu D, Whitcup SM. Effects of the preservative Purite® on the bioavailability of Brimonidine in the aqueous humor of rabbits. J Ocul Pharmacol Ther. 2004;20(4):285-92.
Viscosity-modifying agents increase drug contact time with the ocular surface, thus improving absorption. By raising the contact time, these agents reduce nasolacrimal absorption and improve systemic safety. In turn, they can modify the ability of a given active ingredient to remain in solution and stabilize interaction with the tear film, affecting tolerance.
Therefore, Meseguer et al. (1996)6 conducted a study with human volunteers comparing the corneal contact time of various pilocarpine formulations containing different viscosity enhancers: a phase-transition system (gellan gum, Gelrite®), a heteropolysaccharide (xanthan gum) and different polymers habitually used as control solution: hydroxyethylcellulose, hydroxymethylcellulose, or poly(vinyl alcohol). An eyedrop containing pilocarpine (25 μl) was instilled in one eye only at one-week intervals. The contact time was evaluated by gamma scintigraphy using Technetium-99m (Tc-99m DTPA) as a radioactive label. One minute after instillation, only 23% of the reference formulations remained on the ocular surface, compared with 77% for xanthan gum or 82% for Gelrite)®. Twenty-one minutes after instillation, 12% of the reference solution (polymer), 25% of the xanthan gum solution, and 39% of the Gelrite® solution remained on the ocular surface.
Tabla 2. Comparación estadistica de la actividad residual sobre la superficie ocular de diferentes soluciones oftálmicas que contenían nitrato de pilocarpina al 0,5%. Primer grupo de voluntarios (media+- DE).
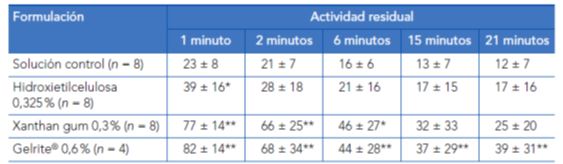
*p<0,05;**p<0,01 (la significación de las diferencias respecto a la solución control se evaluó mediante la prueba de la t de Student).
Fuente: Meseguer G, Buri P, Plazonnet B, Rozier A, Gurny R, Gamma scintigraphic comparison of eyedrops containing pilocarpine in healthy volunteers. J Ocul Pharmacol Ther. 1996 Winter;12(4):481-8.
Dickstein et al. (1996)7 conducted a double-blind, randomized, crossover study to compare the effects of 0.5% aqueous timolol (one drop each 12 hrs) and a 0.5% timolol gellan gum (Gelrite®) suspension (one drop every 24 hrs) on exercise performance in 42 middle-aged men (mean age 58 years, range: 55 to 65 years). Subjects exercised maximally on a cycle ergometer four times with ten-day intervals.
The serum timolol concentrations were 0.91 ± 0.51 ng/ml for timolol solution compared to 0.71 ± 0.46 ng/ml for timolol gellan. The change from baseline in resting heart rate was -1.8 ± 9.3 beats/min for placebo, -11.0 ± 9.6 beats/min for timolol solution, and -8.5 ± 7.5 beats/min for timolol gellan. The change from baseline in peak heart rate was -0.1 ± 7.3 beats/min for placebo, -15.6 ± 5.6 beats/min for timolol solution, and -11.9 ±8.0 beats/min for timolol gellan. These differences are statistically significant.
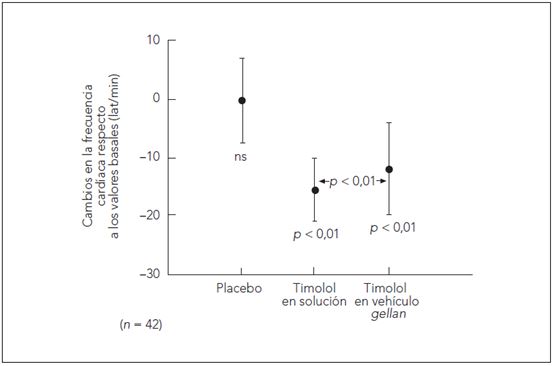
Figura 3. Diferencias en la frecuencia cardíaca (lat/min +-DE) durante el ejercicio. Cambios en la frecuencia cardiaca tras la instilación de placebo, timolol en solución y timolol en vehículo de gellan comparado con la frecuencia basal.
Fuente: Dickstein K, Hapnes R, Aarsland T. Comparison of aqueous and gellan ophthalmic timolol with placebo on the 24-hour heart rate response in patients on treatment for glaucoma. Am J Ophthalmol. 2001;132(5):626-32.
Although both treatments caused reductions in resting and exercise heart rate, timolol gellan was associated with significantly lower reductions. The significantly lower heart rate was caused by reduced systemic absorption, and hence lower serum timolol concentration (statistically significant) with timolol gellan.
Temperature affects active ingredient concentration in eye drops. Kahook et al. (2012)8 conducted a study on the effects of heat in different formulations after 30 days to emulate the shelf life of a package under normal use. The concentrations of different active ingredients (latanoprost, timolol and dorzolamide) were studied, as well as BAK chloride in different brand-name and generic eye drops after exposure to temperatures of 25°C and 50°C for 30 days.
All medications, both brand-name and generic, showed a reduction in active ingredient concentration and BAK when exposed to temperatures above those in the information leaflet. Both Generic latanoprost formulations contained levels of active ingredients that were 10% greater than their labelled value. Generic latanoprost formulations had a significant loss of active ingredient concentration after exposure to temperatures of 25°C and 50°C for 30 days. Generic latanoprost formulations lost more than 10% of the mean active ingredient concentration at temperatures at the high end of the stability limit as stated in the information leaflet, and during their expected time of clinical use. The increased basal active ingredient concentration could be a strategy to compensate the known degradation of drugs over time to levels below their optimal dose, including at room temperature. The timolol-dorzolamide combination in both brand-name and generic medications is relatively resistant to degradation.
Bottles of both generic medications had higher levels of particulate matter compared to brand-name versions. The mean amount of particulate matter significantly increased both in brand-name and generic medications after exposure to 50°C for 30 days. The exact nature of the particulate matter is unknown. Whereas some look solid, others seem to be fibrillary in nature. The origin of particulate matter is unknown and could result from contaminants, active ingredient precipitates or container material. It is not known whether particulate matter can have clinical repercussions on the ocular surface with normal use.
Tabla 3. Número de partículas (por milimetro de volumen) de diámetro superior a 1 µm en el momento basal y después de ser sometidos a estrés térmico
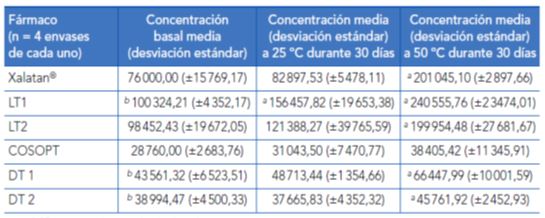
* p < 0,05 comparado con el valor basal.
** p < 0,05 comparado con el valor basal de la marca registrada.
DT, dorzolamida-timolol; LT, latanoprost.
Fuente: Kahook MY, Fechtner RD, Katz LJ, Noecker RJ, Ammar DA. A comparison of active ingredients and preservatives between brand name and generic topical glaucoma medications using liquid chromatography-tandem mass spectrometry. Curr Eye Res. 2012;37(2):101-8.
Generic and innovator medications can also differ in bottle design, viscosity, surface tension and drop volume. Mammo ZN et al. (2012)9 conducted a study to determine whether innovator differed from generic medications marketed in North America in bottle design, viscosity, surface tension and drop volume. Timoptic XE© eye drops from the USA and Canada vary significantly from the generic eye drops Timolol GFS© (USA) and Timolol Maleate EX© (Canada) in drop volume, viscosity, surface tension and bottle tip diameter.
The authors concluded that Timolol Maleate EX© (Canada) and Timolol GFS© (USA) release approximately three-fifths and two-thirds, respectively of the daily therapeutic dose when compared to the country-specific reference medication Timoptic XE© and argued that for a generic medication to be considered interchangeable with the reference medication, inactive components and preservatives in a medication should follow stricter regulatory standards.
Tabla 4. Dosis terapéutica media por fármaco
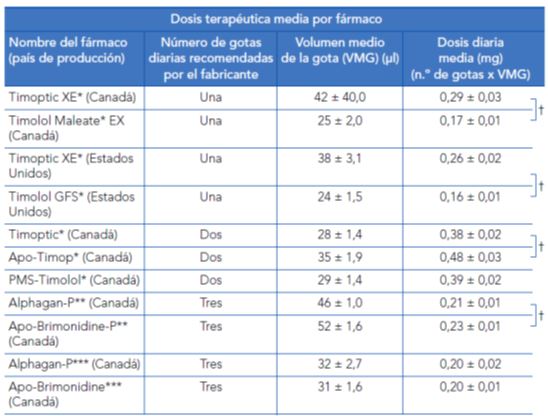
* Timolol maleato solución al 0,5% (6,8mg/ml).
** Brimonidina tartrato al 0,15% (1,5 mg/ml).
*** Brimonidina tartrato al 0,2% (2 mg/ml).
t Los corchetes a la derecha de la tabla indican una diferencia significativa entre las medias (ANOVA) (p < 0,01).
Fuente: Mammo ZN, Flanagan JG, James DF, Trope GE. Generic versus brand-name North American topical glaucoma drops. Can J Ophthalmol. 2012;47(1):55-61
Clinical differences between generic and brand-name anti-inflammatory ophthalmic medications
Corneal complications were seen after instillation of diclofenac post-operatively after routine ophthalmic procedures. A class effect was considered10. The complications were eventually associated with a generic of a certain brand, which was rapidly withdrawn from market11. Which medication component was responsible for corneal complications was not reported.
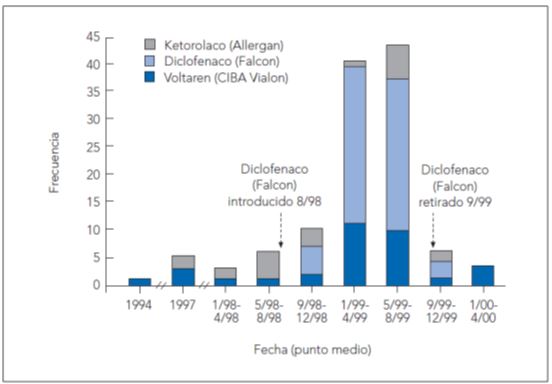
Figura 4. Evolución en el tiempo de todos los casos confirmados de patologia corneal asociados con la administración de antiinflamatorios no esteroideos tópicos.
Fuente: Congdon NG, Schein OD, von Kulatja P, Lubomski LH, Gilbert D, Katz J. Corneal complications associated with tropical ophthalmic use of nonsteroidal antiinflammatory drugs. J Cataract Refract Surg. 2001;27(4):622-31.
Prednisolone acetate is a lipophilic drug, and therefore the original preparation (PredForte®, Allergan) was specifically created as a suspension. The 1% prednisolone acetate suspension requires the patient to shake the bottle in advance to homogenize the suspension. One problem with the generic drug suspension manufactured by Sabex Pharmaceutical (Quebec) is that it would require at least 70 shakes to obtain an homogenous suspension12. Another generic from Falcon Laboratories (Forth Worth) caused occlusion of the eye drop bottle tip potentially due to a prednisolone acetate precipitate12. Upon instillation of the generic eye drops Econopred Plus (Alcon), shaken five times, the active ingredient concentration in each drop was significantly lower compared to the branded medication; about 10% of its maximum concentration compared to 47% in PredForte®13.
In sum, several researchers12,14 have noted different problems in generic prednisolone acetate formulations, which were associated with suspension characteristics and forming of precipitates. Among these, are changes in suspension homogeneity, occlusion of the eye drop bottle tip and significantly lower concentration of the active ingredient in each drop.
Genéricos de fármacos oftálmicos antiglaucomatosos
Antiglaucomatous ophthalmic drug generics
In the absence of comparative studies between the brand-name and the generic medication, FDA classified the generic formulation of the timolol maleate gel forming solution (Falcon Laboratories) as AB, although the products are formulated with different slow-release gel vehicles. Corneal contact time is different for both medications, as well as inactive excipients and preservative concentrations.
Stewart et al. (2002)15 conducted a 12-week randomized, crossover study of Timoptic XE® in comparison with the generic medication in 32 patients. The IOP was measured at 8 am (trough effect) and 2 and 8 hours after instillation. No statistically significant differences were observed between both medications at 8 am, nor 2 hours after administration. However, 8 hours after instillation the IOP was 17.5 ± 3.2 mmHg in the Timoptic XE® group and 18.9 ± 3.3 in the generic medication group (p = 0.0019). Both safety and vision recovery time were similar in both groups.
Narayanaswamy et al. (2007)16 conducted a 24-week randomized, crossover study in India. Patients with ocular hypertension or open-angle glaucoma were treated with a latanoprost generic marketed in India (Latoprost® marketede by Sun Pharmaceuticals) or with reference latanoprost (Xalatan® marketed by Pfizer Ophtalmics). A total of 30 patients were included. During the initial 12-week period, 12 patients were treated with Xalatan®, while the remaining 18 patients received Latoprost®. In the second period, from weeks 13-24, patients initially treated with Xalatan® were switched to Latoprost® and patients who initially received Latoprost® were switched to Xalatan®.
At the end of the first treatment period, patients treated with Xalatan® showed a reduction from baseline in IOP of 23.64 ± 3.13 mmHg to 14.29 ± 1.61 mmHg (a 39% decrease). In patients treated with Latoprost®, the IOP was reduced from 22.74 ± 2.47 mmHg to 16.98 ± 2.49 mmHg (a 25% decrease). In both groups, all patients showed 12-week IOP values lower than 21 mmHg, i.e., they achieved values within the normal range. At the end of the second period, patients changing from Xalatan® to Latoprost® had 24-week IOP values of 15.36 ± 1.71 mmHg and those who switched from Latoprost® to Xalatan® had IOP values of 16.09 ± 1.49 mmHg. There were no significant differences in the incidence of conjunctival hyperaemia or of any other adverse effect.
Tabla 5. Presion intraocular (en mmHg) en ambos grupos en la duodécima semana
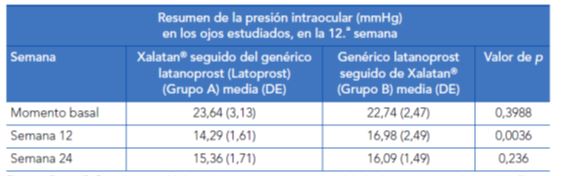
Fuente: Desai C, Rajadhyaksha V. A randomized, crossover, open label pilot study to evaluate the efficacy and safety of Xalatan in comparison with generic Latanoprost (Latoprost) in subjects with primary open angle glaucoma or ocular hypertension. Indian J Ophthalmol. 2007;55(2):127-31.
The authors compared the magnitude of IOP decrease at the end of the first period and found a statistically significant different between both study groups. At the end of the second period, there were no significant differences between both treatments.
The generic medication had a higher pH and higher particulate matter concentration. Such differences may potentially affect both stability and active ingredient release in the eye.
Strmen et al. (2010)17 conducted a randomized, comparative study in the Czech Republic; it was published in Slovakian and only the abstract is available in English. The efficacy in IOP reduction of a new latanoprost formulation (Unilat) is compared with Xalatan®.
Seventy-seven patients were studied and the primary efficacy endpoint was the mean IOP decrease, whereas the second efficacy endpoint was the percentage of patients with IOP value below 21 mm Hg at the end of the study. As reported by the authors in the abstract, the study showed that the new product is non-inferior to the original product.
What will be the repercussions on the daily practice of changing antiglaucomatous medications to generics?
Noecker and Simmons (2010)18,19 consider that although generic product manufacturers comply with all FDA standards, the response of individual patients to a given medicine is unknown. While some patients tolerate eye drop switches with minimal discomfort, others are extremely sensitive and can report small changes associated with preservatives, pH, tonicity or other components. This may lower compliance or lead to several adverse effects.
In general, patients try the medicine we prescribe. If it causes discomfort or adverse effects, patients stop using the medicine and often do not report this until the following check up. On occasions, adverse effects translate into objective signs, but sometimes they appear over time as vague systemic complaints.
The authors consider that substituting an original medicine for a generic medicine could be a trial and error process19. Therefore, the time between follow-up visits should be reduced when patients switch to generics to ensure that the new medicine is working adequately and the patient has no complaints.
To the typical, expectable question: "Doctor, my physician/my pharmacist/my insurer told me I have to switch to a generic. Is it as good as the branded medicine?"
Noecker and Simmons19 adopt different positions according to the patient's clinical status:
1) The patient is newly diagnosed with glaucoma and it is early
in the disease stage. In this case, we are inclined to suggest that the patient try the generic.
2) In patients with advanced glaucoma, especially if they are well-controlled and have previously tried multiple medicines, we are reluctant to suggest a switch. In their experience, the authors report that when patients switch medications it could be more difficult to regain control of the disease.
Cantor (1997)20 considers that when switching to a generic medicine, neither the patient not the physician can be sure that the new medicine will be well tolerated or effective. This author underlines that as doctors we cannot know whether the generic product shows a similar or different eye penetration compared to the branded product. When patients ask about replacing a branded product with its generic counterpart, all we can honestly say as doctors is that the new generic medicine has not been studied, analysed or compared to the original medicine, and so we cannot know whether it will act in a similar way – in other words, whether both medicines are equally effective. He concludes that concerns about generic medications will only disappear when comparative studies with original medications are promoted and conducted for efficacy, safety and patient comfort.
Noecker and Simmons19 state that "There is no doubt we are moving toward a totally generic world, and as our patients adapt to different medicines, we must monitor efficacy and safety vigilantly. This is not to say patients should not use generic drugs, but we must monitor the effects to ensure our patients are getting the cost savings they expect without sacrificing efficacy or creating side effects and tolerability issues."
Finally, some authors consider that if the main and only advantage of generic medicines is their cost, cost analyses should not compare just the medicine price, but they should also bear in mind the following factors: 1) Drop size and bottle fill; 2) Convenience and comfort; 3) Impact on compliance; 4) Potential extra visits or procedures; and 5) Long-term uncertainty.
*The current article was adapted from the book by the same author published by
Editorial Glosa, S.L. (Barcelona)
ISBN: 978-84-7429-572-6
¿Son iguales los genéricos que los fármacos de marca?
Pedro Corsino Fernández Vila
