Do we have other therapeutic weapons besides reducing intraocular pressure?

Nuno Lopes, MD, FEBO
I believe we do.
Glaucoma is a chronic, multifactor neurodegenerative disease of retinal ganglion cells (RGC) and their axons. Traditionally, glaucoma treatment is based on reducing intraocular pressure (IOP).
However, we know that glaucomatous neuropathy can progress despite low, optimized IOP values1.
Since this is the main cause of irreversible blindness in the world2, no efforts have been spared to find adjuvant therapies to reduce the IOP, despite difficulties involved in showing results. The growing understanding of the disease and its etiopathogenesis has facilitated the study of new therapeutic targets implicated in disease progression.
The main areas assessed have been ocular perfusion pressure, translaminar pressure and the neuroprotection, neurorecovery and neuroregeneration triad3.
1. Ocular Perfusion
A number of studies associate hypoperfusion with glaucomatous damage4. However, it is known that eye diseases which primarily involve vascular mechanisms, such as diabetic retinopathy and vascular occlusions, do not show the typical excavation found in glaucomatous damage5,6. Therefore, hypoperfusion cannot be considered a risk factor on its own. The process triggering glaucomatous damage seems to be dependent on a dysfunction of local self-regulation mechanisms, which are incapable of offsetting transient perfusion instability and perpetuate an ischaemia-reperfusion mechanism generating O2 free radicals and inducing the RGC apoptotic cascade7.
Consequently, a significant therapeutic value is attributed to improvement of perfusion pressure and local self-regulation mechanisms8.
2. Translaminar Pressure
Translaminar pressure (TP) is the gradient between IOP and cerebrospinal fluid (CSF) and retrobulbar tissue pressure9,10.
Today, it is recognized that gradient changes influence optical nerve pathophysiology by11,12,13,14,15:
- a mechanical effect of the lamina cribosa bulging with compromise of the axons, support cells and vascular supply
- a reduction in retrograde axonal transport.
Studies have shown significantly lower CSF pressure in subjects with normal-tension (NTG) and primary open-angle (POAG) glaucoma than in controls. Higher TP gradients were also found in NTG patients compared with POAG patients. Parallel studies have also shown a significantly higher CSF pressure in patients with ocular hypertension without any evidence of glaucoma, compared to the control group16,17,18 .
Such results demonstrate barotrauma in NTG cases and suggest there is therapeutic value in increasing CSF pressure within the constitutive range.
3. Neuroprotection, Neurorecovery and Neuroregeneration
The term neuroprotection is quite often used to describe, lato sensu, actions of3:
- Neuroprotection – relative preservation of RGC structure and function
- Neurorecovery – complete or partial restoration of living, but dysfunctional RGCs to a state of structural and functional health, also described as neuroenhancement19
- Neuroregeneration – partial or total generation of new neurons
Even though multiple drugs have already demonstrated in vitro "neuroprotective" properties, none of them has been able to show indisputable evidence of neuroprotection in randomized, controlled studies in large samples20.
Multiple RGC death mechanisms have been studied for the development of neuroprotective strategies, including20,21,22:
- Deprivation of Neurotrophic (NT) Factors – due to axonal transport blockage
- Glutamate Excitotoxicity – distressed RGCs accumulate and release glutamate, which in excess becomes toxic and activates N-methyl-D-aspartate (NMDA) receptors and the apoptotic cascade
- Oxidative Stress – Excess free radicals damage protein structure, DNA and indicate cell death
- Apoptotic pathway – Drugs and gene therapy capable of reducing the expression of caspases, preponderant in cell death cascade, have been studied
- Protein denaturation – common to several neurodegenerative diseases, including glaucoma. Stress or heat-shock proteins facilitate renaturation and protect the three-dimensional structure of proteins from degradation
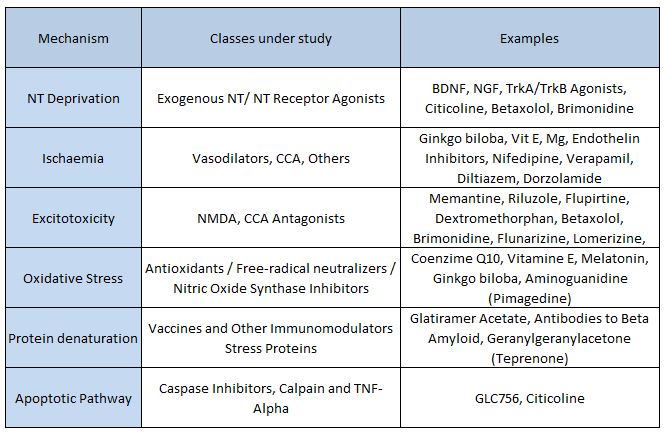
NT; Neurotrophins; BDNF: Brain-derived neurotrophic factor; NGF: Nerve growth factor; TrK: Tyrosine kinase receptor; CCA: Calcium channel antagonists; NMDA: N-methyl-D-aspartate; TNF: Tumour necrosis factor
Table 1. Summary of some damage mechanisms and therapies under study. Adapted from “Neuroprotective Agents in Glaucoma Therapy: Recent Developments and Future Directions”, by B Chua and I Goldberg, Expert Rev Ophthalmol. 2010;5(5):627-636.
The "neuroprotective" properties of some drugs already used for glaucoma treatment have also been studied:
- Betaxolol: has demonstrated a neuroprotective effect in animal models, possibly by preventing glutamate excitotoxicity damage22,23, but a role in increased BDNF synthesis has also been attributed to betaxolol.
- Brimonidine: The LoGTS25,26 (Low Pressure Glaucoma Treatment Study), a multicentre, randomized, double-masked study showed that brimonidine alone preserves visual function better when compared with timolol alone in patients who do not develop allergy. The mechanism may be associated with a beneficial effect of brimonidine or a prejudicial effect of timolol. A possible neuroprotective effect, improved 24-h IOP control (in the study, IOP was not measured at night), improved ocular perfusion pressure, compliance or other unmeasured or unknown variables may have contributed to this result26. Potential neuroprotective mechanisms of brimonidine include increased BDNF levels, activation of anti-apoptotic genes and excitotoxicity inhibition19
- Dorzolamide: This topical carbonic anhydrase inhibitor increases arterial flow velocity27, reduces intracellular pH and prevents apoptosis28. However, study results are conflicting and have not shown a protective effect in animal glaucoma and ocular hypertension models29,30
More recently, Citicoline has also appeared on the glaucoma market supported by studies with experimental evidence of a protective and regenerative effect on RGCs, as well as improved performance in perimeter and electrophysiological tests31,32,33,34. The mechanism of action seems to be mediated by activation of structural phospholipid and neuronal membrane biosynthesis, increasing brain metabolism and neurotransmitter availability35. There is also experimental evidence of a reduction in the number of apoptotic RGCs and an increase in their regeneration, possibly mediated by suppression of caspase 3 and 9 expression31,34.
In light of the above, what can we offer our patients in daily practice besides IOP reduction?
- Accurate ophthalmic examination, including gonioscopy in a dim light environment and generation of a nictemeral curve, when appropriate
- Documentation and broad study of each vascular risk factor and their optimization
- Narrow blood pressure control within recognizably safe values, preventing nocturnal hypotension with an adequately dosed hypotensive therapy or increased salt intake or low dose mineralocorticoids8
- Avoidance of oral carbonic anhydrase inhibitors, especially in NTG patients because of a relevant effect of ICP reduction besides IOP reduction. For translaminar pressure improvement, strategies to raise CSF pressure are not recommended based on current evidence
- Therapeutic adjuvants for glaucoma cases progressing with low pressure:
- Ginkgo Biloba
- Citicoline
- Calcium Channel Inhibitors? (Consider hypotension risk)
- Promotion of a healthy lifestyle according to current knowledge
- Do not underrate non-adherence36 and long-term IOP fluctuation37,38 in patients with POAG progressing with apparently low IOP
- Monitor and motivate to therapy adherence, taking the opportunity to raise awareness among and inform the next generations
- Remember the adjuvant role of techniques unrelated to therapy adherence, with evidence of daytime and long-term IOP fluctuation control:
- Trabeculoplasty39,40: the selective technique does not have the limitations and side effects of traditional technique
- Surgery41 : today, there are less invasive techniques which are safe in reducing the absolute values of and changes in IOP, even in patients suffering from advanced glaucoma to whom we would hesitate to suggest standard techniques
- Commitment to research new adjuvant therapies, using the latest scientific advances in gene therapy, stem cell manipulation, and others so that functional rehabilitation may become a reality in glaucoma clinical practice as soon as possible.
